Global Courant 2023-04-27 05:51:49
Patients and doctors applaud the news that Canada could eventually have a national breast implant registry, describing it as a major step for public safety, but saying it is long overdue.
At a meeting on Tuesday, Mr The Standing Committee on Health of the House of Commons heard submissions for a study on the feasibility of establishing a nationwide registry. The news follows years of advocacy by women who have been harmed and joint investigations by CBC, Radio-Canada and the Toronto Star into the potential dangers of the implants, including cancer and autoimmune diseases.
Julie Elliott, the Canadian representative of the Breast Implant Safety Alliance and spokesperson for the Breast Implant Failure and Illness Society of Canada, said a registry containing data on all implants in this country is long overdue.
“For me, it’s something that should have happened from the time breast implants were produced in 1964,” she told CBC.
She argues that it is strange that foreign objects are placed in the human body without patients and their doctors being able to detect any problems with those devices.
“If you have a car and…something has happened to this car, there’s a recall, you automatically get an email or card telling you to go to your dealer to fix that problem,” said Elliott.
The federal feasibility study will look at what information could be included in a national registry and how it might function once it’s in place.
Health Canada spokesman Mark Johnson said the federal agency will consider the commission’s study and provide a formal response at that time.
“Any next step regarding a breast implant registry would depend heavily on its purpose, structure and design,” Johnson wrote in an email.
He described a breast implant registry as a “complex undertaking” that would require participation and coordination from multiple levels of government.
‘No one is willing to take responsibility’
Some proponents argue that a national registry detailing every implant performed in Canada is needed to ensure that researchers and patients can track illnesses, injuries and other information.
Nancy Pratt says there have been calls for something like this since the 1990s.
Pratt, the BC-based president of the Breast Implant Failure and Illness Society, suffered permanent damage after the breast implants she received in the 1990s ruptured. She later learned that her implants had been recalled, but no one had informed her.
“Everybody makes money off breast implants and nobody is willing to take the responsibility of tracking them down,” Pratt said.
As recently as January or February of this year, she has been hearing from women who have made similar discoveries to hers.
“Women are still finding out that their implants were recalled in 2018, 2019 — and they had no idea,” Pratt said.
She added that any registry should not be limited to implants alone, but should include other materials such as mesh and clips that can also be placed inside the body during implant surgery.
“No one should have a device that has been recalled or has a safety alert on it and they’re not aware of it,” Pratt said.
BC plastic surgeon Dr. Peter Lennox is the former president of the Canadian Society of Plastic Surgeons. (Lennox Cosmetic Surgery)
News of a possible registry came as a surprise to Dr. Peter Lennox, a clinical professor of plastic surgery at the University of BC
He recalled contacting Health Canada in 2017 to recommend setting up a registry, in his role as a member of the executive branch of both the Canadian Society of Plastic Surgeons and the Canadian Society for Aesthetic Plastic Surgery.
“No progress has been made. We had multiple conference calls and meetings with them, and no resolution was forthcoming,” Lennox said.
Other countries are already doing it
Lennox said he is pleased to hear that a registry is finally coming, although there are many questions that need to be answered first, such as whether reporting is required, who runs the database, who has access to it and how it is funded.
“There are several other countries that have very robust breast implant registries and a lot of research has been done on what constitutes a good registry,” he said.
“They don’t have to reinvent the wheel.”
One of those registries is in the Netherlands, where Dr. Jan Willem Cohen Tervaert started studying complications caused by breast implants in the 1990s.
Cohen Tervaert, now a professor of medicine at the University of Alberta, said a series of scandals involving the implant manufacturers have necessitated registrations.
“It is clear that the medical device industry and especially the breast implant medical device industry needs more oversight, and the authorities are failing to do so,” he said.
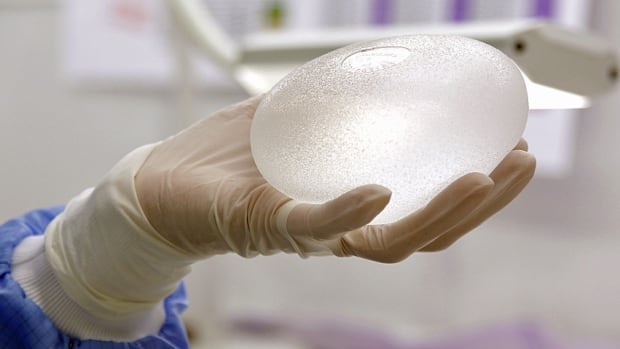
A plastic surgeon holds a breast implant. A registry would let patients know if they have any implants that have been recalled for safety reasons. (Craig Chivers/CBC)
He pointed to the example of Poly Implant Prothèse, a French company that became known in 2010 to sell implants made of industrial-grade silicone rather than the mandatory medical-grade product. The founder of the company ended up in prison for fraud.
“The problem there was that no one had a registry of implants. So even though it was said that the implant had to be removed, no one knew which patients had these implants,” says Cohen Tervaert.
He noted that in the Netherlands, the cost of the registry is now paid by patients, who pay the equivalent of about $40 if they have surgery. The registry operates on an opt-out basis, which is mandatory unless patients choose not to include their data in the database.